SODA-WATER, strange to say, contains no soda at all in any form. As made at present, it is simply a solution of carbonic acid in water of the ordinary purity. It is true that a small proportion of the bicarbonate of soda is sometimes added, but no satisfactory reason is assigned for the practice, and it is believed to be a rare exception to the general rule.
Our readers who have not cared to remember much of their chemistry may need to be reminded that carbonic acid is, at ordinary temperatures and pressures, a colorless gas of slightly pungent odor and pleasant acid taste; and that it exists in abundance in nature, both free and locked up in combination with basic elements, forming carbonates. These carbonates can be easily decomposed, and the carbonic acid obtained from them at will in a pure state.
Water impregnated with this gas is a grateful and, according to the medical authorities, a wholesome drink, although the gas itself, when respired, is fatally poisonous. All fermented beverages which are “brisk,” or sparkling, owe that property to the presence of carbonic acid.
The simplest way of carbonating water is to dissolve in it a soluble carbonate and a decomposing acid. The old-fashioned soda-powders are an application of this principle, the blue paper containing bicarbonate of soda, and the white one tartaric acid, which being dissolved in separate portions of water and the solutions mixed, a brisk effervescence ensues from the escape of liberated carbonic acid. It is reasonable to infer that the carbonated water now known as soda has its name from this extemporaneous method of preparing it. The tartrate of soda formed by the reaction is necessarily present in the liquid, and in order to make pure carbonated water the gas must be allowed to escape from the liquid in which it is set free, and then conducted into pure water contained in a separate vessel. This is essentially what is done in the manufacture of the soda-water of the present day.
At the ordinary pressure of the atmosphere water will absorb carbonic acid only to the extent of a volume equal to its own bulk, which is not nearly a sufficient proportion to give agreeable “soda.” An increase of pressure, however, causes further absorption in a direct ratio, and by taking advantage of this fact water may be acidified to almost any degree desired.
The original method of making soda was to generate the gas and conduct it to a gasometer or gas-holder in the ordinary way, and then force it into the water by means of a condensing air-pump. This method is still in almost exclusive use in England.
In this country the pump is usually dispensed with. The tremendous elasticity of the gas itself when freed from the bonds of chemical union furnishes ample power for compression, and by generating it in a closed vessel of sufficient strength an indefinite number of volumes may be condensed into one, and water impregnated accordingly.
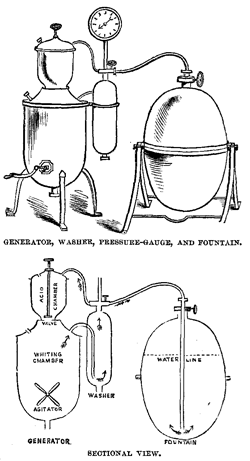 The essentials of an apparatus for making soda-water by the last-mentioned process are a generator and fountain. The generator consists of a strong metallic vessel, divided into two compartments by a diaphragm, or of two separate chambers, mounted one over the other. The upper chamber is a receptacle for the acid to be used, and the lower one receives the carbonate to be decomposed. An opening between the two is furnished with a valve which can be opened or closed at pleasure by an outside connection. The external openings of both vessels are closely fitted with screw plugs, so that after the chemicals are introduced the generator can be rendered gas-tight. The fountain is a cylindrical vessel, into which the water is introduced, and in which it is impregnated with the gas under pressure, and retained until it is drawn for use.
The essentials of an apparatus for making soda-water by the last-mentioned process are a generator and fountain. The generator consists of a strong metallic vessel, divided into two compartments by a diaphragm, or of two separate chambers, mounted one over the other. The upper chamber is a receptacle for the acid to be used, and the lower one receives the carbonate to be decomposed. An opening between the two is furnished with a valve which can be opened or closed at pleasure by an outside connection. The external openings of both vessels are closely fitted with screw plugs, so that after the chemicals are introduced the generator can be rendered gas-tight. The fountain is a cylindrical vessel, into which the water is introduced, and in which it is impregnated with the gas under pressure, and retained until it is drawn for use.
To the generator is attached a small cylinder, which is partially filled with water, through which the gas is conducted to free it from impurities before it reaches the fountain. A gauge, constructed to show the amount of expansive power or pressure exerted by the liberated gas, completes the apparatus. The capacity of the fountains varies from ten to thirty gallons, and that of the generators in like proportion.
The material of which the apparatus is usually constructed is copper, which, from its great toughness and ability to resist expansive pressure, is admirably adapted to the purpose. The acid chamber has a lining of lead, and the fountain one of tin, both of which are necessary to protect the copper from the chemical action of the liquids these vessels are to contain. Fountains of iron lined with glass or enamel are also in use. They afford absolute protection of the water against any metallic contamination, but have the disadvantage of being more weighty, and consequently more troublesome to handle, than copper ones, and are also less secure against explosion.
The chemicals usually employed by the soda makers for the evolution of carbonic acid are whiting or marble dust — both native carbonates of lime, varying only in purity or in aggregation of particles — and sulphuric acid, the oil of vitriol of commerce.
The manufacture of soda-water by the apparatus just noted is exceedingly simple. The process is conducted in the following manner: The whiting, previously mixed with water enough to form a thin, pasty liquid, is introduced into the whiting chamber of the generator through an opening made for the purpose, and the vitriol is in like manner introduced into the “acid chamber.” The washer and fountain each being filled to about two-thirds of its capacity with water, the necessary connection of pipes being made, and the openings secured with well-adapted screw plugs, the acid is allowed to trickle slowly down into the whiting by opening the valve at the bottom of the chamber. The mixture of vitriol and whiting is then thoroughly stirred together by means of an “agitator” operated by a crank on the outside of the chamber, and carbonic acid is rapidly evolved. When the pressure gauge shows a sufficient amount of gas to be present, the cock communicating with the fountain is opened, and the gas flows into it, passing first through the water in the washer, which absorbs impurities that may be brought over from the generator. When the pressure has been equalized in all the vessels, the fountain is disengaged and agitated to promote absorption of the gas by the water; it is then reconnected with the generator, and a fresh charge of gas formed and passed into it, which operation is repeated until the gauge finally shows a uniform pressure against the inner surface of the apparatus of 120 to 160 pounds to the square inch. Sometimes it is carried even higher.
It will be perceived that there is considerable danger attending the manufacture of soda-water. Frightful explosions sometimes occur from the carelessness of the operator, or unnoticeable defects in the apparatus. The water being sufficiently charged with gas, the fountain is connected by a suitable pipe with the draught stand on the counter, where the soda is to be served to customers. As the gas absorbed under pressure rapidly escapes from the water when restraint is removed, and in its efforts to do so forcibly carries with it the water in which it is entangled, the soda is readily “drawn” from the delivery pipe, whatever may be the level or position of the reservoir or “fountain.”
As a summer drink soda is naturally wanted cold, and, with very few exceptions, its lovers “take sugar,” which is supplied in the shape of well-flavored sirups. Accordingly the soda counter must be furnished with apparatus for readily refrigerating the water and for adding the sweets. The former is accomplished either by passing it through a coil of pipe surrounded by ice, or by shaving the ice into the tumbler to be used, which is done by passing it over an inverted plane made for the purpose. The feathery mass thus produced dissolves almost instantly, cooling the soda as soon as drawn. The sirups were in primitive times served simply from a set of appropriate bottles. This method, though still in use to some extent, has been vastly improved on by disposing them in cans or holders provided with faucets, from which they can be drawn with the greatest facility and ease.
The draught stand in its simplest form is merely a pipe rising from the counter to a convenient height, bent to a half circle, so as to deliver the liquid downward, and provided with a stop-cock to regulate the flow at will. The complete draught stand combines in itself the soda draught pipe, refrigerator, and sirup reservoirs. Such an apparatus frequently receives the appellation “fountain,” it and the reservoirs being included in the one title.
In the first efforts toward such an arrangement metal seems to have been exclusively employed, and for a time all the stands were of some silver-plated material. Many beautiful, elaborate, and costly designs were executed in this way. Then marble began to be thought a more elegant material for the purpose than silver, and the taste for it has grown so rapidly that at the present day little, if any, new work is found in metal. From a simple square box, as it were, the form of the marble stand has been developed into new shapes of beauty. One of the most elegant and popular styles is the “cottage,” fashioned after the model indicated by its name. Whatever the form, the arrangement is essentially the same. The sirup cans are concealed within the case, and deliver their varied contents through projecting faucets, and the soda is, of course, delivered in the same manner. Another and a very novel method of serving the sirups is to deliver them all, and also the soda-water, through one pipe, which is done by forming all the necessary connections inside the fountain, and using an ingeniously contrived combination faucet outside, which communicates at will with any compartment in the interior. The cooler consists, as before mentioned, of a coil of pipe, or, what amounts to the same thing, a series of chambers surrounded by ice, through which the soda passes in being drawn. In one form of apparatus an admirable ice-cutter is substituted for the cooler. Its construction is such that by the simple turning of a wheel the ice is drawn against a revolving drum armed with knives or “bits” set plane fashion. The ice, cut into thin feathery shavings, passes through to the inside of the drum, and falls into the vessel placed to receive it. The use of the ice-cutter, which is, of course, only an improved form of the ice-plane, gives the advantage of being able to furnish at will a draught of soda of any required degree of coldness. Soda-water with cream sirups when well iced as above has been fancifully named “ice-cream soda.”
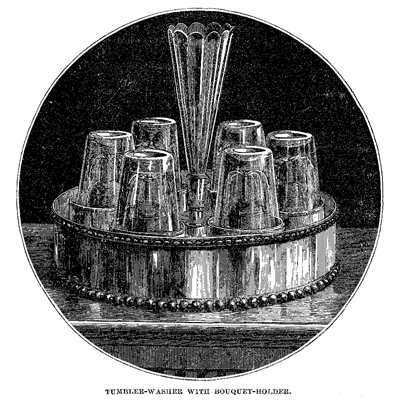 In addition to the convenient and elegant draught stands we have mentioned, the well- appointed soda counter is also furnished with a “tumbler-washer.” The tumbler-washer consists of frames or supports, on which the tumblers are placed in an inverted position.
In addition to the convenient and elegant draught stands we have mentioned, the well- appointed soda counter is also furnished with a “tumbler-washer.” The tumbler-washer consists of frames or supports, on which the tumblers are placed in an inverted position.
In connection with each frame is a jet of water, which is opened by the weight of the tumbler. This jet is so directed that it plays continuously into the inside of the tumbler, and a second one, reversed in position, cleanses the outside. The tumblers are being continually rinsed, and present a delightfully clean and refreshing appearance, which is to be especially appreciated during the dog-days. A “head” of water is, of course, necessary to operate the “washer.”
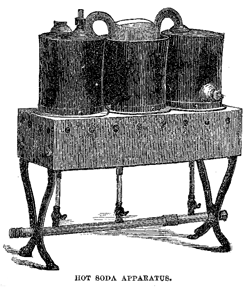 The absence of demand for cold drinks during the winter season has led to the introduction of hot soda, a comparatively recent invention. The new beverage seems to meet with some favor, but its sale amounts to but a mere trifle compared with that of the ice-cold article dispensed in the summer season. In an apparatus for drawing hot soda- water a boiler takes the place of the cooler required in summer. The heat applied is usually a gas flame. The most desirable form of apparatus is one with two or three independent boilers. By such an arrangement a greater or less amount of liquid can be kept ready, according to the demand.
The absence of demand for cold drinks during the winter season has led to the introduction of hot soda, a comparatively recent invention. The new beverage seems to meet with some favor, but its sale amounts to but a mere trifle compared with that of the ice-cold article dispensed in the summer season. In an apparatus for drawing hot soda- water a boiler takes the place of the cooler required in summer. The heat applied is usually a gas flame. The most desirable form of apparatus is one with two or three independent boilers. By such an arrangement a greater or less amount of liquid can be kept ready, according to the demand.
Our notice of soda apparatus would be incomplete without at least a passing mention of the bottling-machine, by means of which carbonated waters can be transferred from the fountain to bottles, and secured therein without any considerable loss of gas. Its mechanism is such that the bottle is held firmly to the nozzle of a draught pipe until filled; the cork is forced in immediately after its removal, and then held firmly in place until permanently secured by wire or twine.
The bottles employed are of two kinds: one somewhat similar in form to a “Champagne,” but of size just sufficient to contain one draught of the water; and a siphon bottle, having capacity for half a dozen glasses or more.
 These “siphons” being fitted with a draught tube which can be readily opened and closed at pleasure by means of a spring valve, any quantity of liquid can be drawn from them as desired without allowing the gas to escape from the portion that remains.
These “siphons” being fitted with a draught tube which can be readily opened and closed at pleasure by means of a spring valve, any quantity of liquid can be drawn from them as desired without allowing the gas to escape from the portion that remains.
By the use of the bottling-machine, soda and mineral waters — i.e., those containing medicinal salts in addition to carbonic acid — are put within the reach of invalids and others who can not go to the” fountain” for a draught.
The sirups, which are an almost universal adjunct of soda-water, are, when of first quality, solutions of the best white (hard crushed) sugar in water, flavored to suit the taste of the consumer.
The flavors most generally used are lemon, strawberry, pine-apple, vanilla, and ginger; but the list is increased almost indefinitely, according to taste or fancy. With hot soda, coffee, or chocolate, flavored sirups have the preference. Lemon is most popular with the ice-cold draught. Fruit sirups, as frequently made, are only a so-called article. The labors of the chemist give us artificial essences which approach near enough in flavor to the aroma of the pine-apple, the strawberry, and a few other fruits to enable us to make excellent imitations of their corresponding sirups. The genuine are better in flavor when well made, but as they are much more difficult to prepare, and spoil more readily than the artificial ones, the latter are likely to be most used by the dealer; and it is doubtful, all things considered, whether the customer is much a loser by the substitution. It must be admitted, however, that delicate stomachs do sometimes detect a difference between the effect of the genuine and the imitation, and that in such cases, at least, the real fruit sirup is to be preferred to the chemical compound.
Genuine fruit juices preserved in sealed bottles are now an article of commerce readily obtainable by those who prefer to make the bona fide sirups.
Unwholesomeness in soda drinks is rather to be suspected in the water itself than in the sirups. When kept in imperfectly lined copper fountains, or drawn through lead instead of tin pipes, it is liable to be contaminated to a serious extent with poisonous salts of the two first-named metals.
Soda-water was first made about seventy years ago. The credit of the invention is said to be due to Austin Thwaites, of Dublin.
The manufacture of soda-water, and the methods of drawing it, have been vastly improved during the past ten or fifteen years. In few other departments of inventive taste and skill have greater strides been made toward perfection during the period named. American ingenuity seems to deserve the credit of leading the van in this march of improvement, for even the French, with all their skill in matters bibulous and gastronomic, learned something new about drinkables when some enterprising Yankees set up an “American soda fountain” at the great Exposition of 1867. The “soude-Américaine à la crème glacée” was a novelty to the Parisians and their guests, and met with a hearty welcome from all.
A Paris paper, noticing the “great success in the potable line,” said:
“It is really one of the curiosities of the Exposition to watch the representatives of every nation on the face of the globe as they make a first trial of the new beverage. The crowd is so great that they are formed in line by the police, and, first securing checks, take a drink in turn. As many as 4000 glasses have been sold in one day, much to the satisfaction of the parties in charge. The contrast between the soda as served in the American style and the eau gazeuse of the French café is so decided as to make the permanent introduction of the former a certainty.”
The manufacturers of the old style of apparatus are, of course, loud in their denunciations of the new. They say that the gas never fully parts with the sulphuric acid it brings over from the generator unless it be allowed to expand in a “rising bell,” as in their machines. As to the truth of their objections we are unable to judge; but whether valid or not, the new arrangement, being so much more convenient than the old, continues to win favor abroad as well as at home. We find from examination of the catalogue of a prominent manufacturer that the American soda apparatus is now in use not only in Europe, but has found its way to far-off Australia, and even to China.
The soda trade is confined chiefly to drug stores, at least in this country — a singular fact, to be accounted for only by the chemical nature of the invention. With praiseworthy good taste the dealers in soda-water vie with each other in the introduction of elegant and attractive apparatus and fittings. The continually increasing (and highly commendable) desire for artistic beauty, as well as convenience in the furniture and fixtures of shops and stores, is scarcely any where more visible than at the soda counter. Amounts of money sufficient to establish a small drug store in the country are expended by city pharmacies on the single item of a draught stand, and nearly as much more is invested in the machinery to manufacture the soda, and for other accessories. Modest outfits, quite plain but neat, can be gotten up for a few hundred dollars, but stores of any pretensions usually spend three or four times as much on this part of their business. Unusually fine draught stands cost as much as $2000. 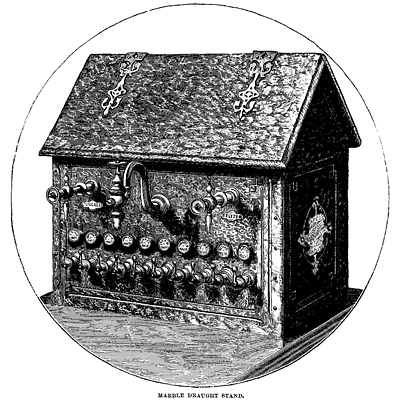 The largest one we have seen described is that of a New York house, which is furnished with the proper appliances for drawing thirty-two different kinds of sirup and eight kinds of “mineral water” in addition to the regular soda. This monster requires eighty gallons of sirup to fill all its cans, and delivers its varied contents through two hundred and fifty feet of conducting and cooling pipe. Late catalogues give estimates of cost for still larger stands.
The largest one we have seen described is that of a New York house, which is furnished with the proper appliances for drawing thirty-two different kinds of sirup and eight kinds of “mineral water” in addition to the regular soda. This monster requires eighty gallons of sirup to fill all its cans, and delivers its varied contents through two hundred and fifty feet of conducting and cooling pipe. Late catalogues give estimates of cost for still larger stands.
It might be inferred from what we have said concerning the liberal, if not extravagant, investments made in the soda business that it was eminently a profitable one; but unless the location is very good, the returns are not nearly so large as might be expected. The expense of operating a fountain is much higher, in proportion, in a small business than in a large one. Proper location is almost every thing in this instance. Although soda-water is so popular a beverage, yet experience proves that the most attractive fountains often fail to draw paying custom when only a little out of the way.